Research on MECP2 Duplication Syndrome has taken an unprecedented step: entering the world of clinical trials. This is a spark of hope for many families who have worked tirelessly for years to provide a better future for their children.
With the launch of early-stage clinical trials such as ATTUNE (ION440 by Ionis) in the U.S. and HERO (HG204 by HuidaGene) in China, we are witnessing a historic moment in the search for a treatment. These phase 1/2 trials will help determine whether these investigational drugs are safe and potentially effective – but they are only the beginning.
To prepare for what comes next – including larger, later-phase trials – we launched a global survey in March 2024.
Its goal is to identify interest and readiness among families worldwide, so we can help support the next steps in trial design, planning, and site selection if current trials show promising results. The survey gathered responses from 94 families across multiple continents.
We would like to sincerely thank all the families who took the time to participate. Every response helps us gather valuable information from the community – and ultimately, to better support the community.
We invite you to explore the results of the survey summarized below.
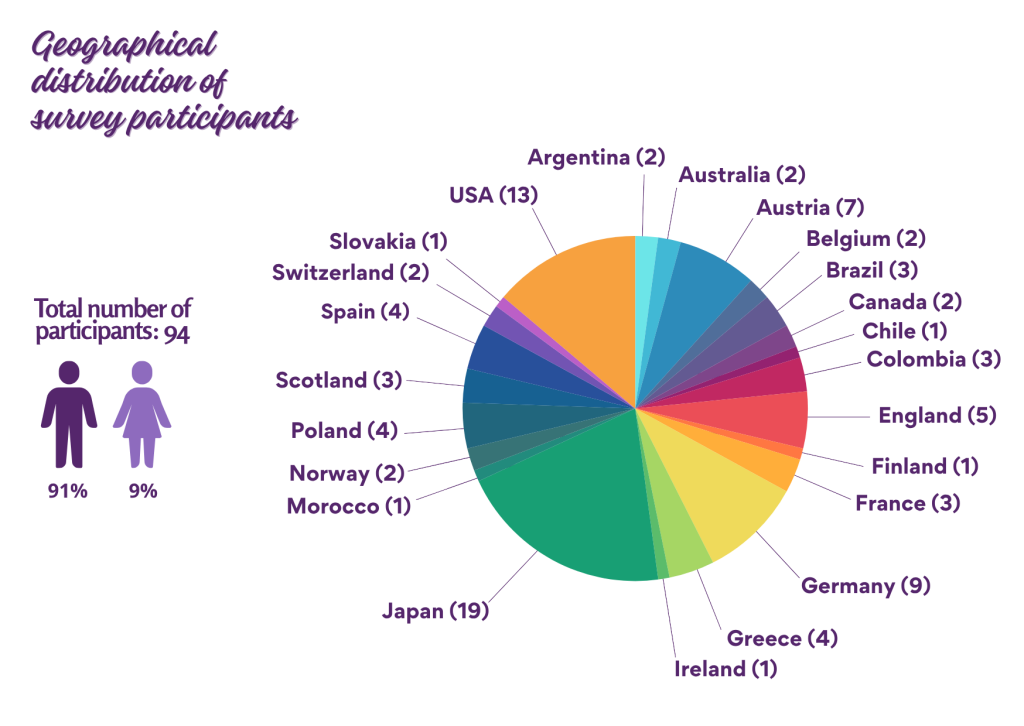
A total of 94 families from around the world participated in the survey – showing strong global interest in participating in clinical trials. The majority of responses came from Europe and North America. Overall, 91% of participants were caregivers or parents of boys, and 9% were caregivers or parents of girls. The children represented in this survey range in age from 0 to 34 years old.
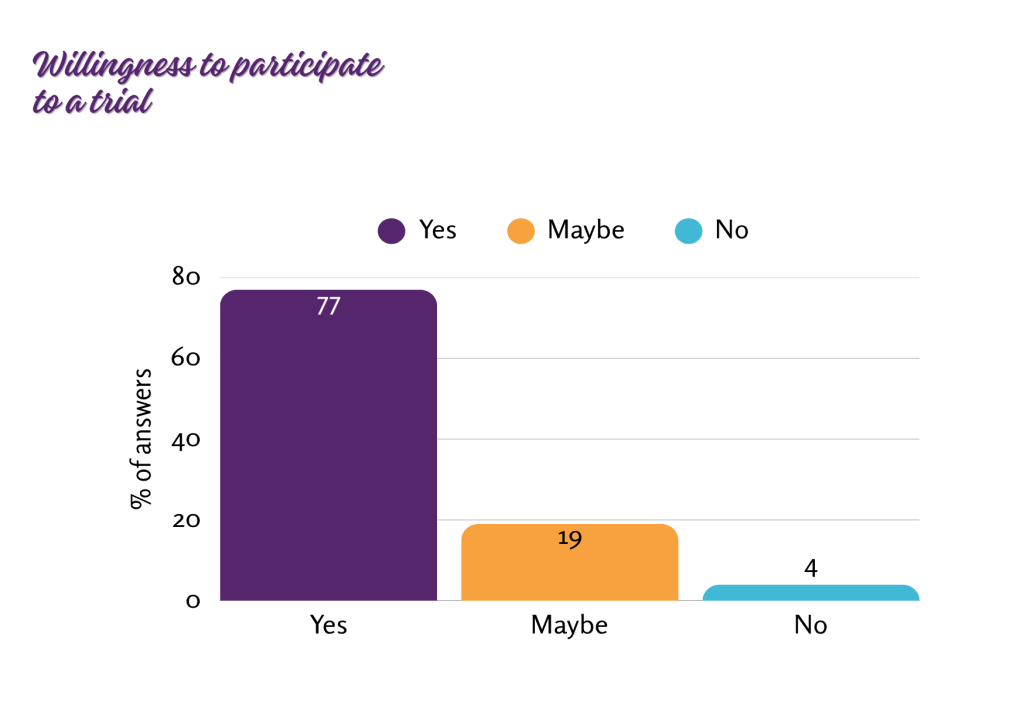
Regardless of sex, age, or geographical distribution, the majority of respondents (77%) expressed strong interest in enrolling their child in a clinical trial. Meanwhile, 19% were undecided, and 4% did not wish to participate.
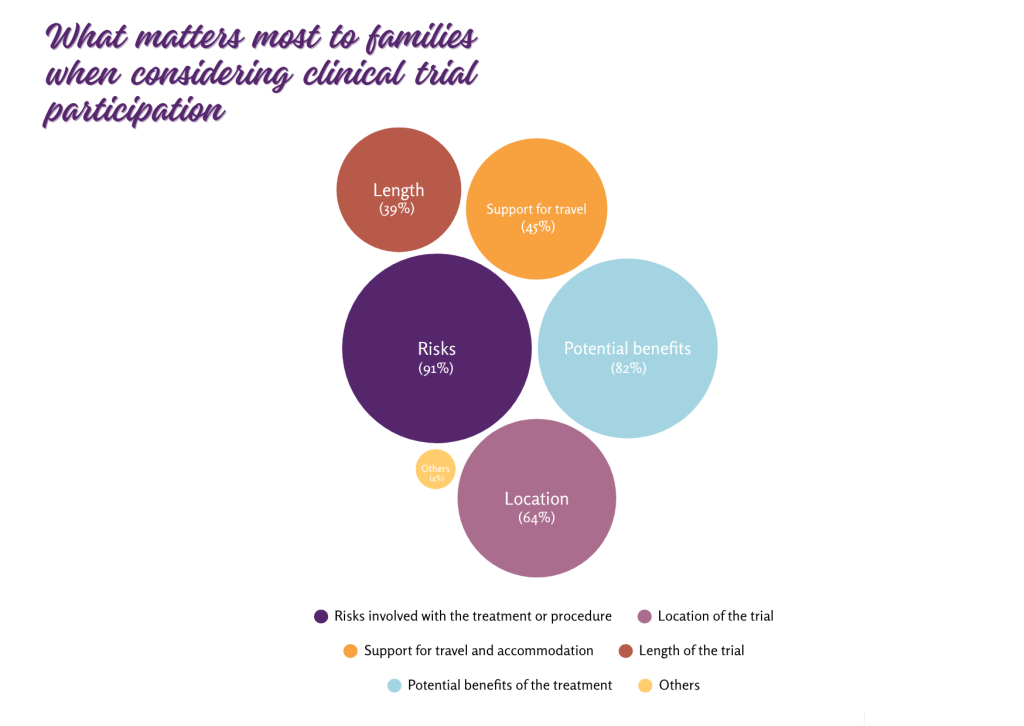
To better understand what influences this interest, families were asked to identify the key factors they consider when deciding whether to enroll their child in a clinical trial. The most frequently selected concern was the risk involved in the treatment or procedure (chosen by 86 out of 94 respondents, 91%). The potential benefit of the treatment followed closely behind (77 out of 94 respondents, 82%). Location of the trial (60 responses, 64%), support for travel and accommodation (47 responses, 50%), and trial duration (37 responses, 39%) were selected less frequently.
Conclusion
The results of this survey provide valuable insights into the perspectives and priorities of families affected by MECP2 Duplication Syndrome.
Despite differences in age, sex, and geography, a strong majority of families expressed interest in participating in clinical trials.
Risk and potential benefit were the most important factors guiding decision-making, while logistical aspects like location, travel, and trial duration – though less critical – remain important considerations.
While ATTUNE and HERO trials are currently collecting first-in-human data, we are hopeful that some of these questions and concerns will be addressed over time.
In the meantime, our community must already prepare for what comes next. If early trial results are promising, broader participation will be essential for later-phase studies and global access. This survey was designed to support that preparation – by understanding where families are, what support they need, and how willing they are to take part.
This survey is more than just data; it is a reflection of a committed and globally connected community, ready to take the next steps toward progress.
For families who have not yet participated, the survey is still open. It’s never too late to make your voice heard and help shape the future of clinical research.
